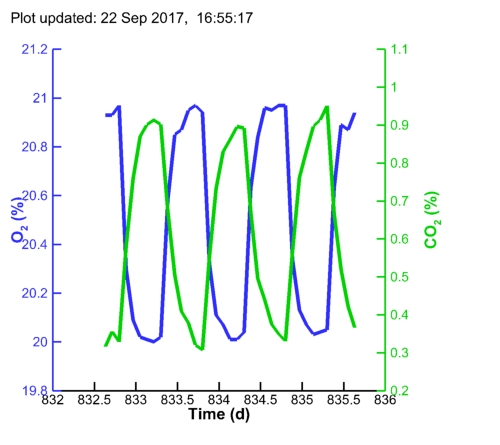
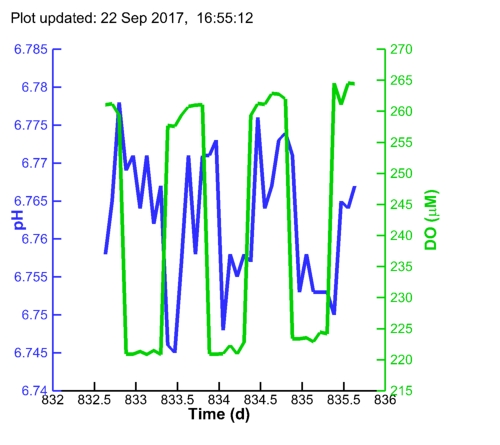
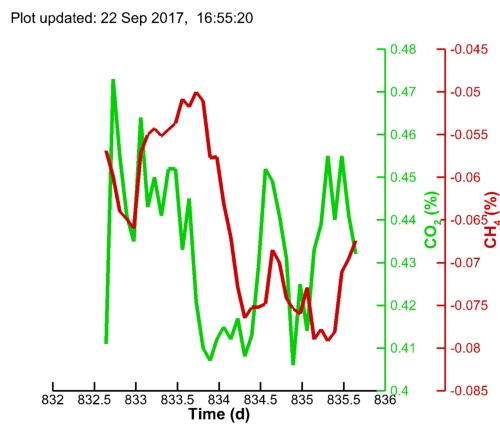
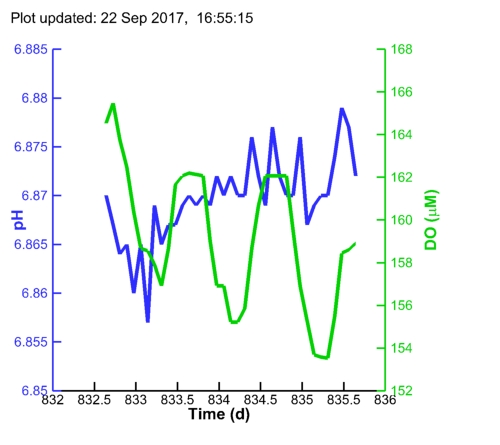
| Algal pH: |
Trace_2015-07-10_17h17_pH_242122-2096.txt,
Trace_2015-07-24_10h01_pH_242122-2096.txt,
Trace_2015-08-01_19h41_pH_242122-2096.txt
Trace_2016-02-22_18h32_pH_242122-2096.txt
Trace_2016-04-29_13h02_pH_242122-2096.txt
Trace_2016-09-16_13h41_pH_242122-2096.txt |
| Algal DO: |
Trace_2015-07-10_17h17_DO_242453-02-210052.txt,
Trace_2015-07-24_10h02_DO_242453-02-210052.txt,
Trace_2015-08-01_19h42_DO_242453-02-210052.txt,
Trace_2016-02-22_18h32_DO_242453-02-210052.txt
Trace_2016-04-29_13h03_DO_242453-02-210052.txt
Trace_2016-09-16_13h41_DO_242453-02-210052.txt |
| Digestor pH: |
Trace_2015-07-10_17h17_pH_242122-2100.txt,
Trace_2015-07-24_10h02_pH_242122-2100.txt,
Trace_2015-08-01_19h42_pH_242122-2100.txt,
Trace_2016-02-22_18h32_pH_242122-2100.txt
Trace_2016-04-29_13h03_pH_242122-2100.txt
Trace_2016-09-16_13h41_pH_242122-2100.txt |
| Digestor DO: |
Trace_2015-07-10_17h17_DO_242453-02-210053.txt,
Trace_2015-07-24_10h02_DO_242453-02-210053.txt,
Trace_2015-08-01_19h42_DO_242453-02-210053.txt,
Trace_2016-02-22_18h32_DO_242453-02-210053.txt
Trace_2016-04-29_09h57_DO_242453-02-210053.txt
Trace_2016-09-16_13h41_DO_242453-02-210053.txt |